Pfizer Biontech Impfstoff
We aspire to utilize the full potential of the immune system to fight cancer and infectious diseases. Have had a solid organ.
Biontech Pfizer Deal Mit Ioc Impf Spende Fur Olympia Zdfheute
In einer gemeinsamen Pressemitteilung äußerten sich BioNTech und Pfizer zum Markennamen des Impfstoffs.
Pfizer biontech impfstoff. Der Impfstoff wird in der EU unter dem Markennamen COMIRNATY vermarktet der eine. Seite 3 von 3 kbv steckbrief impfstoff comirnaty von biontechpfizer 19. In South Africa where the SARS-CoV-2 variant of concern B1351 beta was predominant 100 95 CI 535 1000 VE was observed.
BioNTech and Pfizer are jointly developing BNT162. Comirnaty Pfizer is approved for use in people aged 12 years and over. It is recommended to administer the second dose 3 weeks after the first dose see sections 44 and 51.
You may access the full guidance document here. A two-dose regimen of BNT162b2 30 μg per dose given 21 days apart was found to be safe and 95 effective against Covid-19. Global Information About PfizerBioNTech COVID19 Vaccine also known as BNT162b2 The approval status of the PfizerBioNTech COVID19 Vaccine varies worldwide.
The TGA provisionally approved it for use in Australia on 25 January 2021 for 16 years and over and 22 July 2021 for 12 years and over. What you can do to stay safe and prevent the spread. Der Impfstoff des deutschen Herstellers Biontech und seinem US-Partner Pfizer ist in den USA bereits für Kinder und Jugendliche ab zwölf Jahren und für Erwachsene zugelassen.
Funded by BioNTech and Pfizer. First COVID-19 vaccine approved for children aged 12 to 15 in EU. Safety over a median of 2 months was similar to that of other viral vaccines.
Lipp Chefapotheker des Universitätsklinikums Tübingen die Vorbereitung des Biontech-Impfstoffes. WHOs Strategic Advisory Group of Experts on Immunization has issued interim recommendations for the use of the Pfizer BioNTech BNT162b2 vaccine against COVID-19This article provides a summary of those interim recommendations. Talk to your vaccination provider if you have questions.
Servicebiontechde You are also welcome to use our contact form. The collaboration with Pfizer is built on the 2018 agreement to jointly develop an mRNA-based influenza vaccine. Home Pfizer Biontech - BiontechPfizer Impfstoff zugelassen.
The effectiveness of the pfizer inc biontech se vaccine in preventing infection by the coronavirus dropped to 47 from 88 six months after. Funded by biontech and pfizer. A two-dose regimen of BNT162b2 conferred 95 protection against Covid-19 in persons 16 years of age or older.
Severely immunosuppressed includes individuals who. BioNTech und Pfizer wollen nach positiven Studienergebnissen. While we continue to see the devastating impact of the coronavirus pandemic around the world we are committed to helping keep people safe and informed.
It aimed to rapidly advance multiple COVID-19 vaccine candidates into human clinical testing based on BioNTechs proprietary mRNA vaccine platforms with the objective of ensuring rapid worldwide access to the vaccine. FDA Paves Way for PfizerBioNTechs COVID-19 Shot In Young Children. BioNTechPfizer-Impfstoff sicher und wirksam bei Kindern.
And our scientific efforts that we hope will help bring an end to the current global health crisis. 49 6131 9084-0 For questions on general topics. Updated 25 June 2021 pursuant to updated interim recommendations.
In diesem Lehrvideo erklärt Prof. More flexible storage conditions for BioNTechPfizers COVID-19 vaccine. BioNTech is collaborating with Fosun Pharma to develop BNT162 in China where the companies expect to conduct trials.
In Australias vaccine rollout the Pfizer vaccine is. Additional manufacturing capacity for BioNTechPfizers COVID-19 vaccine. Pfizer and BioNTech plan to submit the efficacy and safety data from the study for peer-review in a scientific journal once analysis of the data is completed.
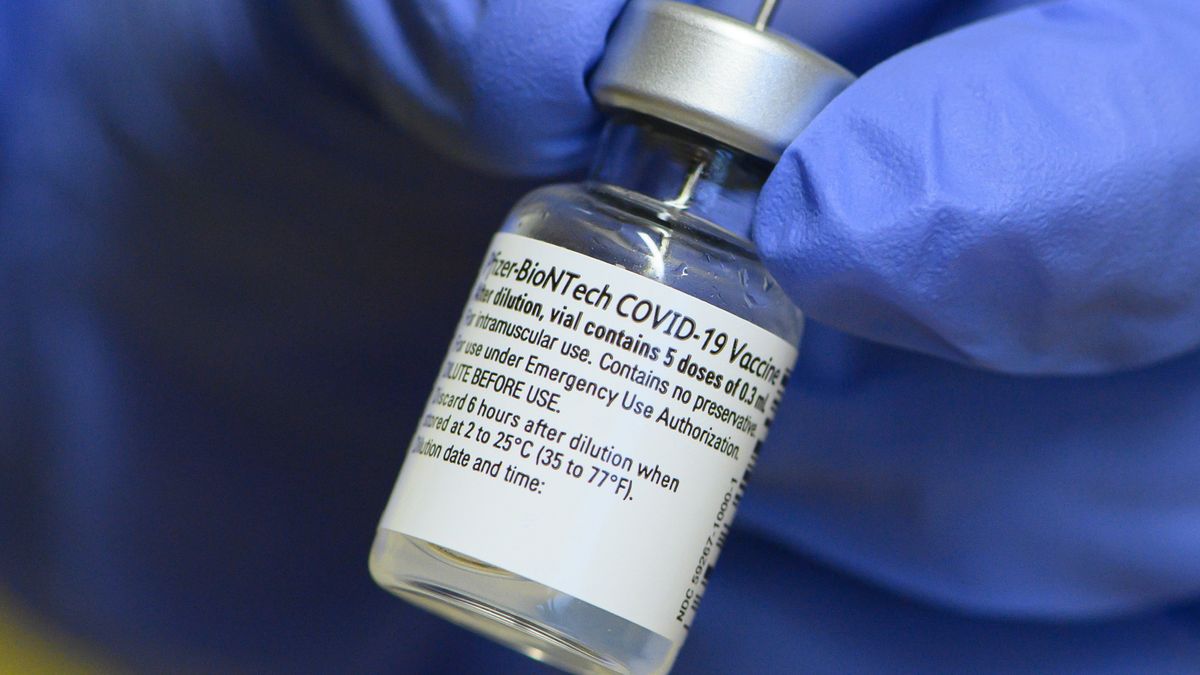
Conclusion With up to 6 months of follow-up and despite a gradually declining trend in vaccine efficacy BNT162b2 had a favorable safety profile and was highly efficacious in preventing COVID-19. The Pfizer-BioNTech COVID-19 Vaccine is a suspension for intramuscular injection administered as a series of two doses 03 mL each 3 weeks apart. During the clinical development stage BioNTech will provide its partners clinical supply of the vaccine from its GMP-certified mRNA manufacturing facilities in Europe.
USA genehmigen Comirnaty für Kinder ab fünf Jahren. In countries where the vaccine has not been approved by the relevant regulatory authority it is an investigational drug and its safety and efficacy have not been established. BioNTech SE An der Goldgrube 12 55131 Mainz Germany.
Learn about SARS-CoV-2 the virus that causes COVID-19. Aug 03 2021 der impfstoff comirnaty von biontechpfizer ist ein hochwirksamer impfstoff gegen das coronavirus. Pfizer-BioNTech COVID-19 Vaccine which you may receive because there is currently a pandemic of COVID-19.
Breakthroughs That Change Patients Lives At Pfizer we apply science and our global resources to bring therapies to people that extend and significantly improve their lives. Letztes Update am Montag 20092021 1107.
Biontech Pfizer Impfstoff Schutzt Vor Coronavirus Mutation Br24

Cominarty Biontech Pfizer Impfstoff Mit Aufgezogener Spritze Mit Einer Impfstoffflasche Cominarty

Comirnaty Biontech Pfizer Impfstoff Comirnaty Biontech Pfizer Vaccine Copyright Xbeautifulxsport

Us Zulassung Von Biontech Pfizer Impfstoff Erwartet Wissen Umwelt Dw 09 12 2020
Over Half Of German Respondents In Favor Of Ending Vaccination Prioritization Newsroom Universitat Hamburg

Coronaimpfung Vollstandige Us Zulassung Fur Impfstoff Von Biontech Pfizer Kleinezeitung At
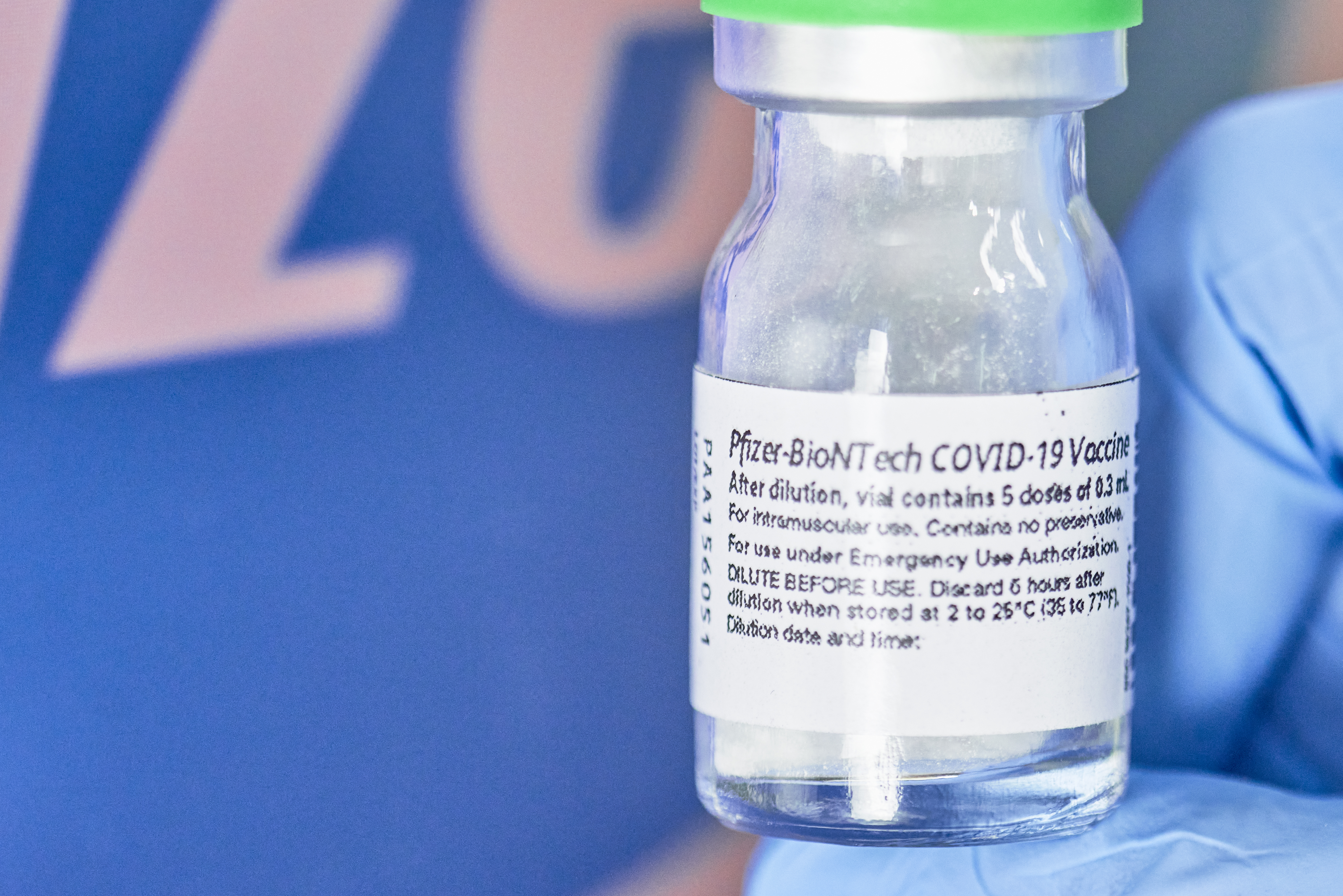
File Pfizer Biontech Impfstoff Ampulle 50744958791 Jpg Wikimedia Commons
Studie Zu Biontech Pfizer Impfstoff Impfung Schutzt Wohl Auch Vor Ansteckung Forschung Lehre

Studie Zur Wirksamkeit Biontech Impfung Schutzt Offenbar Lange Geo

Coronavirus Uk Approves Pfizer Biontech Vaccine To Roll Out Next Week News Dw 02 12 2020

Comirnaty Biontech Pfizer Impfstoff Auf Eis Comirnaty Biontech Pfizer Vaccine On Hold Copyright

Covid 19 Biontech Pfizer Impfstoff Bei Kindern Ab 12 Jahren

Cominarty Biontech Pfizer Impfstoff Mit Aufgezogener Spritze Kochsalzloesung Und Aufkleber Comina

Corona Impfstoff Biontech Pfizer Beantragen Notzulassung In Usa Br24

Coronavirus Germany Hopeful Of Earlier Eu Vaccine Approval News Dw 15 12 2020

Covid 19 Vaccine Is Over 90 Effective Pfizer Biontech Say After Early Data Of Phase 3 Trials Youtube

Eu Behorde Gibt Grunes Licht Fur Corona Impfstoff Von Biontech Br24

Cuba S Covid Vaccine Rivals Biontech Pfizer Moderna Americas North And South American News Impacting On Europe Dw 27 06 2021
